
FDA Cranks up Pressure for Modeling and Simulation
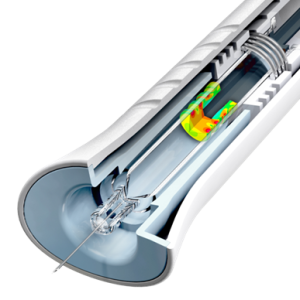
Figure 1: Computational modeling of an elastomeric stopper used in a drug delivery device.
In November 2023, the FDA released new guidance on assessing the credibility of computational modeling and simulation (CM&S) in medical device submissions.
This guidance underscores the agency’s commitment to advancing digital strategies and driving the broader adoption of CM&S in regulatory processes. Despite its transformative potential, the adoption of CM&S based approaches in the medical device sector has been slow.
Barriers to CM&S Adoption in Medical Device Development
While CM&S has been widely adopted in industries like aerospace and automotive, its application in medical device development has been slow. Several key factors contribute to this resistance:
- Regulatory Uncertainty: Physical testing is the gold standard in medical device verification and validation. CM&S, by contrast, lacks a comparable history of standardized validation criteria. Shifting away from traditional physical testing has created uncertainty for manufacturers. There is a concern that reliance on CM&S in a medical device development may add risk to the development timeline, if the FDA does not accept the scientific evidence based on CM&S approaches In the current climate, although the FDA does want to see more use of CM&S in regulatory submissions, it is not likely they will go as far as offering assurances of acceptance. In the end, the work will be judged on its merit, case-by-case.
- Technical Complexity: Developing CM&S tools for medical devices often requires advanced simulations of complex biological and mechanical interactions. These models demand specialized software, high-performance computing infrastructure, and technical expertise, which can be prohibitively expensive for small and medium-sized companies.
- Cultural and Organizational Resistance: Many organizations are accustomed to established workflows centered around physical testing and clinical trials. Shifting to a CM&S approach requires changes to quality management systems, staff training, and risk management practices. This cultural inertia can lead to resistance, as noted in the Reagan-Udall Foundation’s report on in silico technologies.
- Concerns Over Model Credibility: For regulatory approval, CM&S models must be “credible”, i.e. able to produce reliable, accurate results within their intended use. Demonstrating this credibility involves rigorous verification, validation, and uncertainty quantification, which can deter companies unfamiliar with these processes.

The Importance of Embracing CM&S
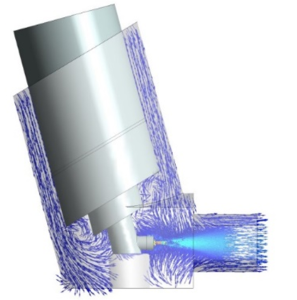
Figure 2: Computational fluid dynamics (CFD) based simulation of the airflow and particle behavior inside an inhaler device.
Despite these challenges, adopting CM&S is no longer optional. The benefits are too significant to ignore:
- Cost Efficiency: CM&S can dramatically reduce the need for physical prototypes and testing for devices with high development costs and complex designs, this efficiency is a game-changer.
- Improved Safety and Risk Assessment: Simulations allow developers to evaluate device performance across a wide range of conditions, including edge cases that may be impractical to test physically. This leads to better risk assessment and enhanced patient safety.
- Accelerated Time to Market: By streamlining design verification testing and other preclinical evaluations, CM&S shortens development timelines. This is particularly beneficial for devices addressing urgent healthcare needs.
- Supporting Health Care Innovation: CM&S enables rapid iteration and optimization, driving advancements in MedTech that improve patient outcomes and enhance the efficiency of clinical trials.
FDA’s Commitment to CM&S Adoption
The FDA’s 2023 guidance represents a clear call to action for medical device manufacturers. By laying out structured pathways for establishing CM&S credibility, the FDA is encouraging companies to adopt computational approaches while reducing regulatory uncertainty. The guidance emphasizes three pillars of CM&S credibility:

- Verification: Ensuring that the model’s underlying mathematics are implemented accurately and consistently.
- Validation: Demonstrating that the model reliably represents real-world phenomena or device performance.
- Uncertainty Quantification: Identifying and addressing sources of uncertainty to build confidence in the model’s predictions.
By addressing credibility concerns directly, the FDA aims to alleviate one of the primary barriers to CM&S adoption. The agency’s endorsement of standards like ASME V&V 40 reinforces that these frameworks are not optional but critical for regulatory acceptance.
Blended Approaches: CM&S and Physical Testing
While the FDA’s guidance highlights the importance of CM&S, it doesn’t suggest abandoning physical testing altogether. A blended approach that combines computational modeling with targeted physical tests offers the best of both worlds. For instance, physical testing can validate CM&S results, reinforcing model credibility. Similarly, simulations can identify scenarios for focused physical testing, reducing the number of tests needed and cutting costs. These synergistic approaches accelerate the iterative design process, ensuring devices meet safety and performance standards efficiently.
Path to Success: Partnering for CM&S Excellence
For companies new to CM&S, collaboration is key. Partnering with organizations, such as SES/Force-4, that are experienced in computational modeling, design verification testing as well as regulatory affairs can bridge knowledge gaps and streamline the transition to digital strategies. [ In Silico Design Verification Testing of Medical Devices – SES Force-4 ] These partnerships offer several advantages:
- Expertise in Model Development: Collaborators bring technical proficiency in creating, verifying, and validating CM&S models tailored to regulatory requirements.
- Regulatory Navigation: Experienced partners understand the nuances of FDA expectations and can guide companies through the submission process.
- Blended Testing Strategies: A partner can help integrate CM&S with physical testing, ensuring a comprehensive approach to device verification and validation.
By leveraging external expertise, companies can overcome initial barriers and unlock the full potential of CM&S in medical device development. SES/Force-4 specializes in helping Medtech innovators adopt computational strategies, ensuring their models meet FDA standards while accelerating time to market.
Looking Ahead: A Digital Transformation in Medtech
As CM&S adoption continues to grow, its integration into regulatory processes will reshape how medical devices are designed, tested, and approved. By combining computational and physical testing, investing in expertise, and leveraging new FDA frameworks, the industry can create safer, more effective devices that meet the needs of patients and healthcare providers worldwide.

Author
Keep in touch with us.
Sign up for our newsletter.


![SES Force-4 is Presenting Virtual ASTM D4169 Testing at the[PACK]out® 2025](https://sesforce4.com/wp-content/uploads/2025/04/thePACKout-Jay-Yuan-400x250.jpg)
