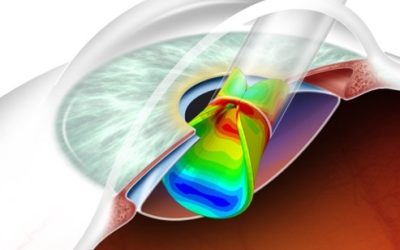
THE CHALLENGE
A case study is presented to discuss Container Closure Integrity (CCI) and the challenges of Novel Pharmaceuticals and Antiquated Containers has reduced design margins and how the industry is currently evolving solutions.
BACKGROUND
To understand the current state of cold storage in this branch of the pharmaceutical industry, it is important to understand that the storage requirements for pre-filled syringes and vials did not instantly transition from room temperature storage to dry ice storage. Such a drastic change would have initiated a paradigm shift in container design, similar to how the cryogenic industry responded. Rather, the storage requirements have gradually shifted through several phases. Each phase of lower temperatures was loosely defined and seemingly settled on by incidents between pharmaceutical companies and container vendors. Neither had a complete grasp on the potentials, hurdles, or impacts of each other’s needs and challenges.
pharmaceutical industry, it is important to understand that the storage requirements for pre-filled syringes and vials did not instantly transition from room temperature storage to dry ice storage. Such a drastic change would have initiated a paradigm shift in container design, similar to how the cryogenic industry responded. Rather, the storage requirements have gradually shifted through several phases. Each phase of lower temperatures was loosely defined and seemingly settled on by incidents between pharmaceutical companies and container vendors. Neither had a complete grasp on the potentials, hurdles, or impacts of each other’s needs and challenges.
This has led to incremental advances where solutions are designed and tested in methods similar to the conventional ways where ‘make it more squishy or compress it more’ and ‘test more’ are the dominating solutions. This approach can work within a certain range, but as it approaches the limits of the materials and design, it fundamentally fails because it is a black-box solution that does not address the physics of the problem.
FINDINGS & INSIGHTS
Storage at 2°C to 8°C
In response to the early onset of colder storage temperatures within the range of 2°C to 8°C, the pharmaceutical industry initially sought to justify prevailing solutions through a rigorous regimen of extensive testing. This rationale asserted that the specified temperatures maintained an ample distance from the glass transition temperature (Tg), a crucial metric denoting the temperature at which a material undergoes a material transition from a rigid to a more pliable state.
While this empirical approach provided a form of verification, it fell short in identifying the proximity of the design to a critical threshold in terms of temperature sensitivity.
For the most part, this approach demonstrated that the current product solutions were valid with some additional manufacturing controls implemented if failures in production were found. For most of these occurrences, additional controls were focused on outlier manufacturing or assembly issues related to geometric tolerances, trim edge quality, or basic material properties like hardness.
Storage at 2°C to -20°C
Subsequently, as storage temperatures descended further to -20°C, the industry continued to lean on the same justification, only to encounter instances of seemingly sporadic failures. The absence of a robust, physics-based comprehension prompted extensive testing programs. Broad arrays of seal and container combinations were tested in large DOE formats as predicated upon the expectation that optimal performance would be derived from just finding the superior configuration.
Concurrently, vendors initiated a phase of experimental endeavors, to introduce various solutions aimed at aligning with the dynamic requirements of the industry. Regrettably, these endeavors often transpired with a myopic insight into the imminent necessity for even colder storage conditions in the near future. The industry’s response to these evolving demands has predominantly manifested in a reactive modality, driven by exhaustive testing rather than the cultivation of an in-depth understanding of the fundamental physics governing sealing processes.
Ultimately, this approach had pockets of success. Many of the elastomers’ onsets of Tg were below -20 °C, and the critical characteristics that changed at these temperatures were minor enough to fall within the current statistical variation. It eliminated a few products that had suboptimal design features that couldn’t provide sufficient seal integrity. It highlighted some of the advantages related to films and lubrication.
Centers of excellence such as Stress Engineering Services, Inc. became more involved through numerous partners and demonstrated the need for some products to improve the tolerances controls related to:
- Geometry
- Manufacturing
- Material elasticity
- Assembly
- Handling
Below -20°C
As some drug products required storage at or below -20°C due to limited shelf life at 2-8°C, the industry started to notice significantly increased risks to CCI, and current solutions were obviously unfit, and more attention was needed. Stress Engineering Services, Inc. worked with several industry leaders and has been active in the community to provide insights into why storage below -20°C is challenging and what attributes must be optimized to succeed.
FURTHER INSIGHTS & DOCUMENTATION
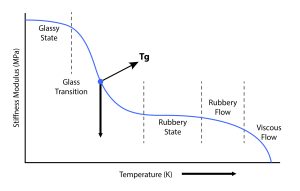
Figure 2 – Glass Transition Temperature Insights
The links below include the first two of three industry presentations providing insights into thinking technically about CCI for PFS in cold storage and treating CCI as a knowable outcome rather than just passing a test.
PDA, 2022, Palm Springs, Jeremy Hemingway
PDA, 2020, Basel, Jeremy Hemingway
AUTHOR
Jeremy Hemmingway, P.E., Head of Drug Delivery Solutions

Reference: The Potential for Chronopharmacology to Revolutionize Patient Outcomes – C009-PDD
At Force-4, Smart Ideas For A Smart World, we are a passionate, multidisciplinary team of expert problem-solvers ready to tackle your most challenging technical problems. We pride ourselves on our innovative solutions, which elevate how businesses operate. Partnering with SES has provided forward-thinking solutions for companies and industries requiring in-depth technical knowledge and proven performance in engineering design and analysis, thermal and fluid sciences, instrumentation, and testing.
Keep in touch with us.
Sign up for our newsletter.