Move more reliable medical devices to market in less time.
The SES/Force-4 Team uses proven alternatives to the traditional build-test-modify-repeat model for developing medical devices that can reduce time-to-market by as much as 25%. Keep in touch with us. Sign up for our newsletter. Email: First Name: Last Name: Submit...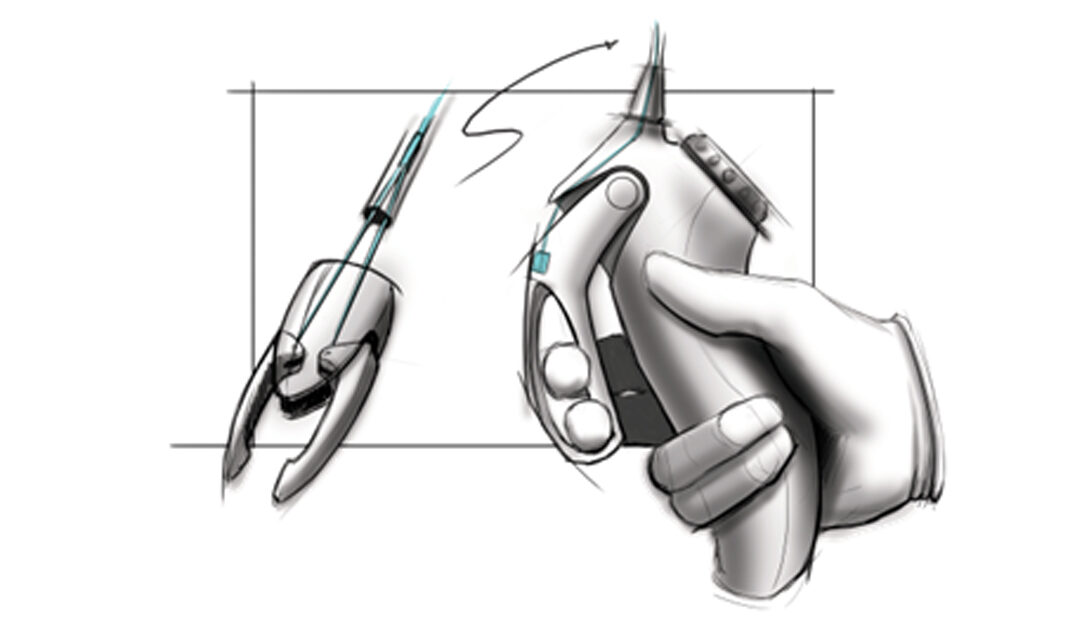
Human factors engineering enables you to design innovative products!
Heuristic Analysis + PCA Analysis + FMEA & FTA + Formative Evaluation + Summative Evaluation Keep in touch with us. Sign up for our newsletter. Email: First Name: Last Name: Submit View Our Innovation...