Pharmaceutical Engineering
The opportunity for innovation in the pharmaceutical industry is constantly evolving. Our development and testing capabilities and ability to synthesize new market demand ensure products meet the emerging needs of patients, clinicians, and caregivers.
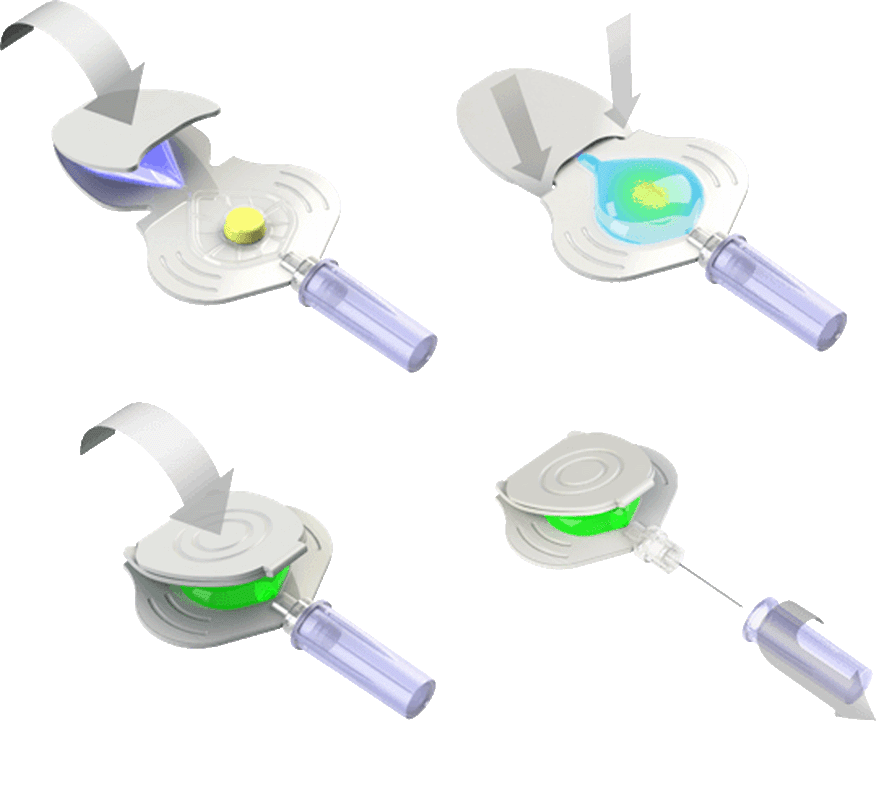
Drug Delivery & Medical Systems Design
Safe, reliable drug delivery is vital for pharmaceutical companies in the hospital or home. SES Force-4 knows the importance of simple, secure, reliable devices and packaging and can solve your most complex design challenges. Our deep knowledge of pumps, syringes, patches, pens, inhalers, and the technology driving innovation in drug delivery will give you an advantage through the approval process and beyond.
Looking Forward
How Will Your Product Perform?
Understanding Real Time Aging means knowing your product will meet or exceed its labeling claims. Learn how SES Force-4 can help you understand your product’s shelf life.
Looking Forward
How Will Your Product Perform?
Understanding Real Time Aging means knowing your product will meet or exceed its labeling claims. Learn how SES Force-4 can help you understand your product’s shelf life.


Enabling Predictive Outcomes
Skip the empirically driven try, fail, and test cycles, which can consume time and resources and too often result in missed opportunities for improved performance and reliability. We partner with your staff and lab to uncover and address technical challenges before they become technical problems, delivering a reliable product to market on time and within your budget.
Pharmaceutical
Case Studies
Featured Content
Case Studies & Industry Stories
FDA Cranks Up Pressure for Modeling and Simulation
FDA Cranks up Pressure for Modeling and Simulation In November 2023, the FDA released new guidance on assessing the credibility of computational modeling and simulation (CM&S) in medical device submissions. This guidance underscores the agency's...
eCommerce: A Match for Sustainable Offerings
eCommerce: A Match for Sustainable Offerings eCommerce has done something monumental in changing consumer consumption patterns that have previously existed for many decades. Retail environments, such as general stores or specialized outlets, have enabled consumers to...
SES Force-4 is Presenting Virtual ASTM D4169 Testing at the[PACK]out® 2025
SES Force-4’s Jay Yuan, PhD, PE, is Presenting ASTM D4169 Virtual Testing at the[PACK]out® 2025 We’re pleased to announce that our own Jay Yuan will present a talk about how virtual ASTM D4169 testing can improve sterile barrier integrity, reduce over-design, and cut...



![SES Force-4 is Presenting Virtual ASTM D4169 Testing at the[PACK]out® 2025](https://sesforce4.com/wp-content/uploads/2025/04/thePACKout-Jay-Yuan-400x250.jpg)
