Medical Device Development
We understand the ups and downs of product development, which is rarely a linear process. Partnering with SES Force-4 early in the development process will transform your concept, advance your innovation, and improve your bottom line.
Human Factored Design
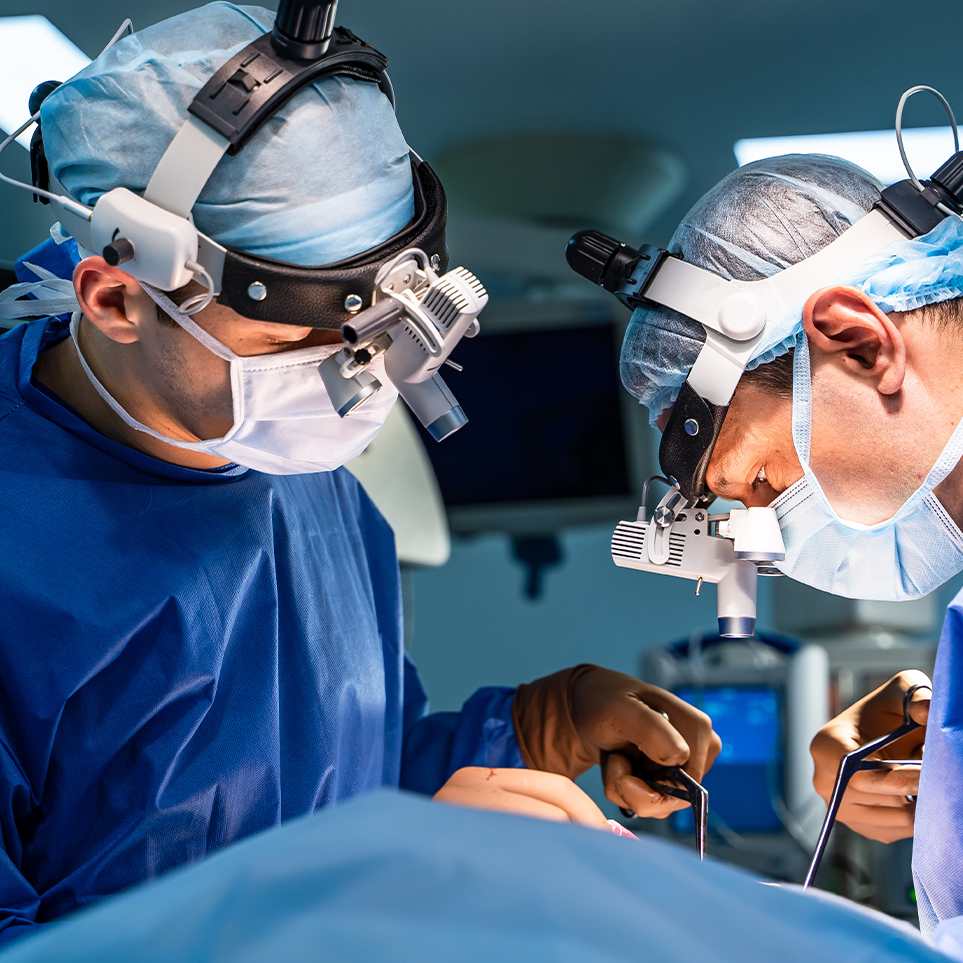
Medical device development demands a broad range of specialized capabilities and expertise. The primary objective of any new technology or device developed is to enhance the lives of patients and caregivers by providing the best outcome. Our team designs and engineers devices that meet that need, plus the diverse needs of surgeons, nurses, and critical care teams. Together, we’re innovating for a better, safer, healthier future.

Looking Forward
ISO 9001:2015 and ISO 13485:201 Certified
Our team will steward your device through the design, development, and rigorous approval process. This commitment to product reliability and patient safety echoes throughout our quality management system (QMS).
Looking Forward
ISO 9001:2015 and ISO 13485:201 Certified
Our team will steward your device through the design, development, and rigorous approval process. This commitment to product reliability and patient safety echoes throughout our quality management system (QMS).
Emerging Technology
The increased use of telemedicine and wearable devices to monitor health and medication is a driving force in the medical device design space. Innovation is at the forefront of developing safe technology that enhances patient care and improves outcomes. Telemedicine providers’ unique needs present unique challenges and benefits for patients and healthcare professionals. Our team has a holistic approach to harnessing the power of today’s emerging technologies for the healthcare industry and beyond.
Medical Device
Case Studies
Featured Content
Case Studies & Industry Stories
FDA Cranks Up Pressure for Modeling and Simulation
FDA Cranks up Pressure for Modeling and Simulation In November 2023, the FDA released new guidance on assessing the credibility of computational modeling and simulation (CM&S) in medical device submissions. This guidance underscores the agency's...
eCommerce: A Match for Sustainable Offerings
eCommerce: A Match for Sustainable Offerings eCommerce has done something monumental in changing consumer consumption patterns that have previously existed for many decades. Retail environments, such as general stores or specialized outlets, have enabled consumers to...
SES Force-4 is Presenting Virtual ASTM D4169 Testing at the[PACK]out® 2025
SES Force-4’s Jay Yuan, PhD, PE, is Presenting ASTM D4169 Virtual Testing at the[PACK]out® 2025 We’re pleased to announce that our own Jay Yuan will present a talk about how virtual ASTM D4169 testing can improve sterile barrier integrity, reduce over-design, and cut...



![SES Force-4 is Presenting Virtual ASTM D4169 Testing at the[PACK]out® 2025](https://sesforce4.com/wp-content/uploads/2025/04/thePACKout-Jay-Yuan-400x250.jpg)
